Customer Hotline:
Customer Hotline:+1 6193853151

 Customer Hotline:
Customer Hotline:+1 6193853151

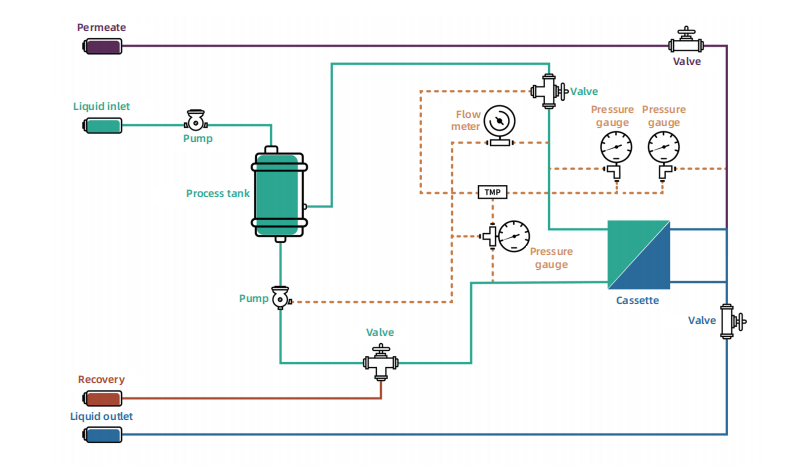
The semi-automatic TFF system adopts a modular design and is available in benchtop and floor-standing models. It is equipped with filter holders of different specifications. According to process requirements, the circulation pump can be flexibly configured with a rotor pump or a diaphragm pump.
The system is designed to be compact and practical. With automatic feedback and adjustment of the flow rate and pressure, the automatic control of TMP and Delta P is available, thereby realizing the automatic operations of concentration, diafiltration, desalting, and dealcoholizing of biological products such as antibodies, vaccines, blood products, and gene therapy drugs.
Due to its automatic operation, cost-effectiveness, and compliance with regulations related to data integrity, the system is an ideal choice for filtration systems in pilot-scale manufacturing, medical product manufacturing for clinical use, and the GMP manufacturing phase for some products, especially for polyvalent pneumococcal vaccines, blood products, gene therapy medicines, and cell therapy drugs.
 Product Model:FiltraLinx® Semi-automatic TFF
Product Model:FiltraLinx® Semi-automatic TFF  Manufacturer nature:Manufacturer
Manufacturer nature:Manufacturer  Update time:2025-06-20
Update time:2025-06-20  Visits:77
Visits:77 Product catalog
Related articles
Detailed Introduction
Available in various specifications: Standard benchtop model for 0.1–0.5 m2 cassettes; standard benchtop model for 0.5–2.5 m2 cassettes; floor-standing models for different cassette sizes, even 200 m2 and above.
PLC, weighing unit, manual valve, circulation pump, flow meter, pressure sensor, supporting feedback control of TMP and Delta P, to facilitate automatic manufacturing.
Compatible with both the ultrafiltration cassettes and hollow fiber filters.
Filtration tank (available in PP and 316L stainless steel) with window, compatible with single-use mixing and storage bags for sample loading and collection.
Feeding pump for equal-volume diafiltration.
Siemens 12-in HMI panel.
Software operation interface in Chinese/English, loaded with Chinese input methods.
Emergency stop button.
Hierarchical alarm.
Three-tier access control.
Comply with the requirements on electronic record and electronic signature management in FDA 21 CFR Part 11.
Comply with audit trail requirements.
Display of process parameter trend charts with reports in PDF format.
Reserved cassette integrity detection interface.
Reserved SCADA system data interface.
Optional instruments: online pH, online conductivity, and online UV.
Optional functions: CIP and water flux detection.

|
Membrane area |
0.1–0.5 m2 |
0.5–2.5 m2 | 2.5–5 m2 | 5–10 m2 | 2.5–20 m2 |
|
Cat. No. |
TS-CMV-S03 | TS-CMV-S20 | TS-CMV-Q40 | TS-CMV-L30 |
TS-CMV-L67 |
|
Automatic level |
Automatic control |
||||
|
Max. operating pressure |
5 bar |
||||
|
Max. back pressure |
6 bar |
||||
|
Tubing |
SS 316L Ra ≤ 0.4 μm |
||||
|
Sanitary connection |
TC |
||||
|
Manual valve |
PP, SS 316L |
||||
|
Pressure detection |
0–6 bar |
||||
|
Flow detection |
Permeate end, electromagnetic flow meter; retentate end, electromagnetic flow meter |
||||
|
Electrical unit |
SIEMENS S7 |
||||
|
Operation mode |
Automatic recording and analysis by software |
||||
|
Operating system |
Win 10 |
||||
|
Operating temperature |
4–40 °C @ PP; 4–60 °C @ SS 316L |
||||
|
Min. circulation volume and residual volume |
Benchtop model for 0.1–0.5 m2 cassettes: min. circulation volume - 200 mL Benchtop model for 0.5–2.5 m2 cassettes: min. circulation volume - 400 mL |
||||
|
Language |
Chinese/English, loaded with Chinese input methods |
||||
|
Material- liquid contact part |
316L, Polymer (USP class VI or FDA), EPDM, Santoprene elastomer, medical, Grade epoxy, silicone |
||||
Hierarchical alarm.
Three-tier access control.
Comply with the requirements on electronic record and electronic signature management in FDA 21 CFR Part 11.
Comply with audit trail requirements.
Display of process parameter trend charts with reports in PDF format.